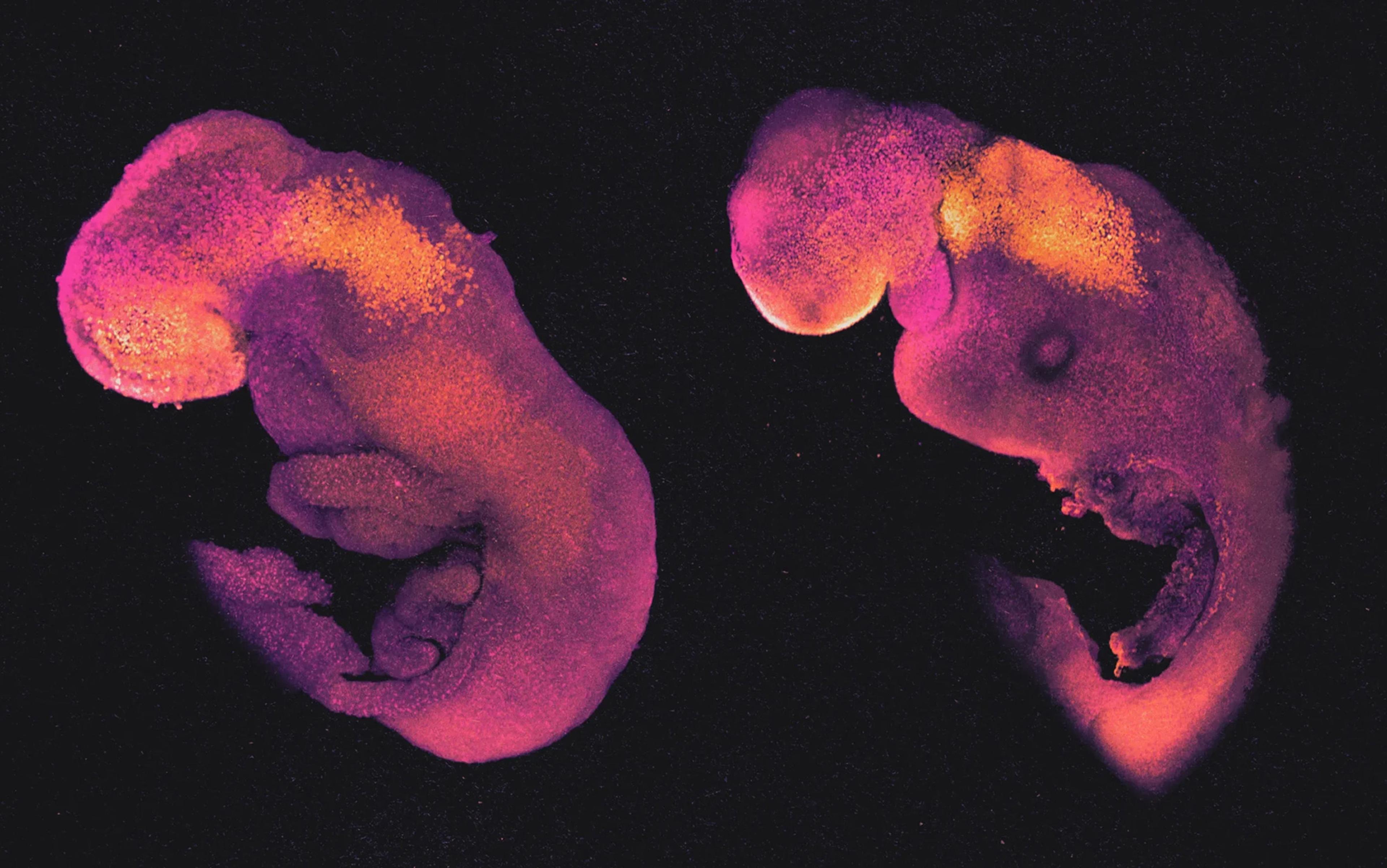
Fifty-four years ago, I did something extraordinary. I built myself. I was a single, round cell with not the slightest hint of my final form. Yet the shape of my body now – the same body – is dazzlingly complex. I am comprised of trillions of cells. And hundreds of different kinds of cells; I have brain cells, muscle cells, kidney cells. I have hair follicles, though tragically few still decorate my head.
But there was a time when I was just one cell. And so were you. And so were my cats, Samson and Big Mitch. That salmon I had for dinner last night and the last mosquito that bit you also started as a single cell. So did Tyrannosaurus rex and so do California redwoods. No matter how simple or complex, every organism starts as a single cell. And from that humble origin emerges what Charles Darwin called ‘endless forms most beautiful’.
Once you’ve come to terms with that mind-boggling fact, consider this: all organisms, including humans, build themselves. Our construction proceeds with no architects, no contractors, no builders; it is our own cells that build our bodies. Watching an embryo, then, is rather like watching a pile of bricks somehow make themselves into a house, to paraphrase the biologist Jamie Davies in Life Unfolding (2014).
This process of body sculpting is called embryonic development, and it is a symphony of cells and tissues conducted by genetics, biochemistry and mechanics. People who study this, like me, are called developmental biologists. And while you may not know it, our field is in a period of tremendous excitement, but also upheaval.
In the summer of 2022, I sat in the back of a lecture hall in Santa Cruz, California listening to a lecture from Magdalena Żernicka-Goetz, professor of mammalian development and stem cell biology at the University of Cambridge, UK. She is a controversial figure and one of many scientists trying to push the limits of understanding human embryos. I heard, too, from Ruth Lehmann, director of MIT’s prestigious Whitehead Institute for Biomedical Research. She’d been in the news for firing a famous scientist for sexual harassment, but what’s made her an international leader in biology for decades is her brilliant and creative study of developmental biology, in fruit flies.
This juxtaposition of fly and human embryos wasn’t surprising; developmental biology is propelled by a whole zoo of embryos – fruit flies, yes, but also sea urchins, worms, frogs, mice. Indeed, our great triumph in the 20th century was revealing the astonishing molecular similarity of all embryos; and, for precisely that reason, studies of animal embryos have garnered seven Nobel Prizes in the past 30 years alone. What surprised me in Santa Cruz was just how fast our collective understanding of animal embryos is making possible truly explosive advances in human embryology. So, while Lehmann’s fascinating new work on cell migration in fly embryos kept the audience rapt, it was Żernicka-Goetz who caught the media’s attention.
Developmental biology is something society needs to understand. And don’t we want to?
Together with Jacob Hanna’s lab in Israel, Żernicka-Goetz was building what scientists call ‘embryo models’. These biological entities look a lot like embryos; they start as relatively few cells and few cell types, and they grow and elaborate over time. But they’re not made in the usual way. Eschewing both egg and sperm, embryo models are created by manipulating embryonic stem cells. Perhaps best known to the public for their promised miracle cures or as proxies for abortion debates, these cells display a remarkable power. They can be made to differentiate into essentially any cell type in the body. Now, it seems, we might even use them to make embryos.
When Hanna and Żernicka-Goetz each published their findings after the meeting in Santa Cruz, The Washington Post wrote that the advances put ‘the possibility of a complete human synthetic embryo on the horizon’. That nomenclature was unfortunate, as these aren’t synthetic at all, but rather entirely biological. (That’s why scientists prefer the term ‘embryo models’.) But they were spot on about the implications. And about the timing: reports of embryo models made from human stem cells hit newspapers exactly a year later, in the summer of 2023.
This is no incremental change and, despite the flawed press narrative, Żernicka-Goetz and Hanna aren’t the only or even the most important players in the game. Other influential biologists are making huge strides too, though their names aren’t often in the press. Some have even argued that the new advances ‘challenge the current legal definitions of the embryo’, which prompts the question: how should we define an embryo? And what do we do when, as they certainly will, scientists’ definitions differ from the general public’s? As embryo models become more sophisticated, how will we know when that clump of tissue in the dish becomes an embryo?
I’ve studied embryos for more than 30 years, and while it doesn’t often catch the public’s attention, developmental biology is something society needs to understand. And don’t we want to? Isn’t it just another way of framing that ancient and universal question: How did I get here?
Human contemplation of embryonic development is nearly as old as writing. In the Old Testament story, Job asks of God: ‘Didst thou not pour me out like milk and curdle me like cheese?’ Half a world away, the Buddha uses the same dairy-based metaphor in the Garbhāvakrāntisūtra, a 1st-century scripture. Some of the earliest cultures in Southern Mexico left no writing, but they made statues of human fetuses. Anywhere you go in the ancient world, you find embryos.
In ancient Greece, as light began to show in the cracks that separate religion, philosophy and science, a remarkable treatise appeared. To modern eyes, On the Nature of the Child – attributed to Hippocrates – is bent on explaining human development, though it does so largely by describing the development of a hen’s egg. Actually, not an egg but 20 eggs, each of which the author exhorts us to open on successive days, so we can observe development over time: ‘You will find everything as I say in so far as a bird can resemble a man.’
Aristotle rejected preformation, and argued instead for a progressive development
That ancient appreciation of time is critical, for it frames the first key question in the history of developmental biology: does an embryo acquire its complexity piece by piece, somehow progressively assembling itself? Or is that new organism already present in the egg or sperm, preformed, as it were, and needing only to be spurred somehow to grow? Some readers will be familiar with the iconic image of preformation – a tiny human curled up inside a sperm. Its late-17th-century printing underscores just how long we struggled to resolve these two poles of thought, progressive versus preformed.
Aristotle himself was the first to weigh in. Consulting farmers and fishermen with the same enthusiasm with which he debated scholars, the philosopher described everything from the live births of dolphins to the size of elephant embryos. He compared the embryos of chickens, fish, insects and, yes, humans. He rejected preformation: ‘our senses tell us plainly that this does not happen’. He argued instead for a progressive development, and while it took 2,000 years to resolve, he was exactly right.
Just how this progression happens remains the core question of developmental biology. And as we begin to explore the truly uncharted morality of embryo models and their progressive development, what strikes me most about the concept is how neatly it parallels ancient thoughts about inchoate humanity.
In the modern debate over abortion, the doctrine that ‘life begins at conception’ is now so constantly repeated that it’s often assumed to have an ancient, perhaps even scriptural origin. It does not.
In fact, in Catholic canon law, the doctrine dates precisely to 12 October 1869, when Pope Pius IX declared excommunication as the penalty for anyone involved in obtaining any abortion. For the nearly 2,000 years that had gone before, however, many Christian thinkers held the embryo to acquire its humanity only gradually. This concept, linked to the ‘animation’ or ‘ensoulment’ of the embryo, arose in laws first set down more than 3,000 years ago that imposed increasingly harsher penalties for causing the loss of a pregnancy as it progressed.
The idea was widely, if not uniformly, adopted by early Christian jurists. St Augustine held this view; St Basil was opposed. None wielded greater influence than St Thomas Aquinas, whose 13th-century rendering of Aristotle’s progressive acquisition of humanity in utero became a prominent, perhaps dominant concept in Western Christianity. It surfaced everywhere from Dante’s poetry to Celtic law for 500 years.
The embryos of scientists are not the embryos of the public, or the Church
Of course, saints weren’t the only ones thinking about embryos. Leonardo da Vinci drew several in the 16th century, one now famous for its inaccuracy. When the modern university was being developed in a 16th-century Italy roiled by Protestant Reformation and Catholic Counter-Reformation, scholars on both sides cracked open chicken eggs to study embryos. A century later, a less divided group (all Royalists in the English civil wars) still hotly debated the chick embryo. And when modern science began to emerge in the 17th century, its founding figures had more than a passing interest in the embryo.
By the 19th century, the new scientists had reached consensus. The concept of progressive embryonic development of animal embryos was established once and for all. But then as now, the embryos of scientists are not the embryos of the public, or the Church. In an odd synchronicity, science and Church staked out opposite views at essentially the same time.
A mere 23 days separated Pope Pius’s decision and an important lecture by the embryologist Wilhelm His. Propounding a new vision for understanding progressive development of the embryo, His would go on to publish The Form of Our Body and the Physiological Problem of Its Development (1874). It was – despite the possessive in the title – a thoroughgoing discussion of chicken embryos. But His said exactly what he meant. Soon after, he would combine lessons learned from chickens with a network of physicians, and become the first to comprehensively define, cogently describe, and accurately display the progressive development of human embryos.
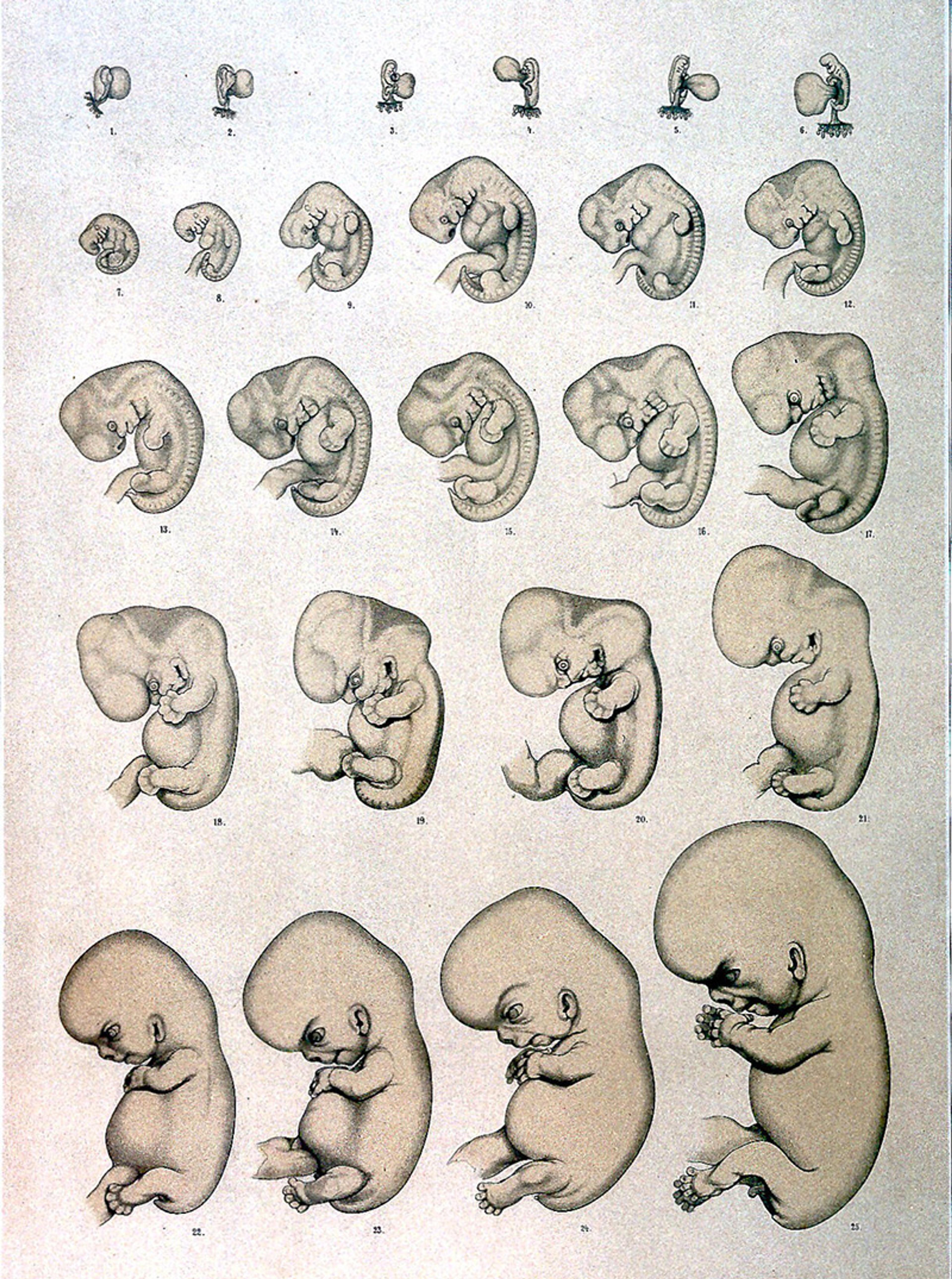
Selections from the ‘normal table’ of human development: the embryologist Wilhelm His et al ‘produced’ the scientific conception of the human embryo in the 1870s using careful staging and illustration. From Anatomie menschlicher Embryonen (1880-85). Courtesy the Wellcome Library
As the Cambridge historian Nick Hopwood put it, His and others produced the very concept of the embryo as we know it. And, while embryos certainly exist as tangible, biological entities, this concept is so central to the work of developmental biologists that we rarely notice it. We’re also slow to consider how others in society relate to it. And that’s important, because, in the 20th century, the concept of the embryo changed radically yet again.
By the time the famous double helix structure of DNA was discovered in the early 1950s, fruit flies like Lehmann’s had taught us that genes direct the inheritance of traits from one generation to the next; sea urchins showed us that genes reside on chromosomes in the cell nucleus; and bacteria and viruses revealed that genes were made of DNA. But the relationship between our genes and our development was still mostly a black box. When we first peeked in, it wasn’t through the ascendant disciplines of genetics and biochemistry, but a more hands-on approach: transplantation. Not of organs, but of cellular bits.
In Nobel Prize-winning work, the British developmental biologist John Gurdon showed that if he destroyed the gene-containing nucleus of a one-cell frog embryo, normal development could be restored by transplanting the nucleus of some other cell. Fascinatingly, any cell nucleus might do the job, suggesting the tools needed to guide development of an entire organism are present in each and every one of its cells.
But there was a catch. Donor nuclei from early embryonic cells were far better at restoring development than those taken from later embryos. Such decreasing ‘potency’ over time was a crucial revelation for understanding progressive development. The concept has its apotheosis in the British developmental biologist Conrad Waddington’s ‘landscape’, an iconic image depicting an early embryonic cell as a marble set to roll down a branching network of increasingly deep valleys. At the top, the marble might still roll into any number of valleys, but its inventory of potential shrinks with its descent. It can’t roll back uphill.
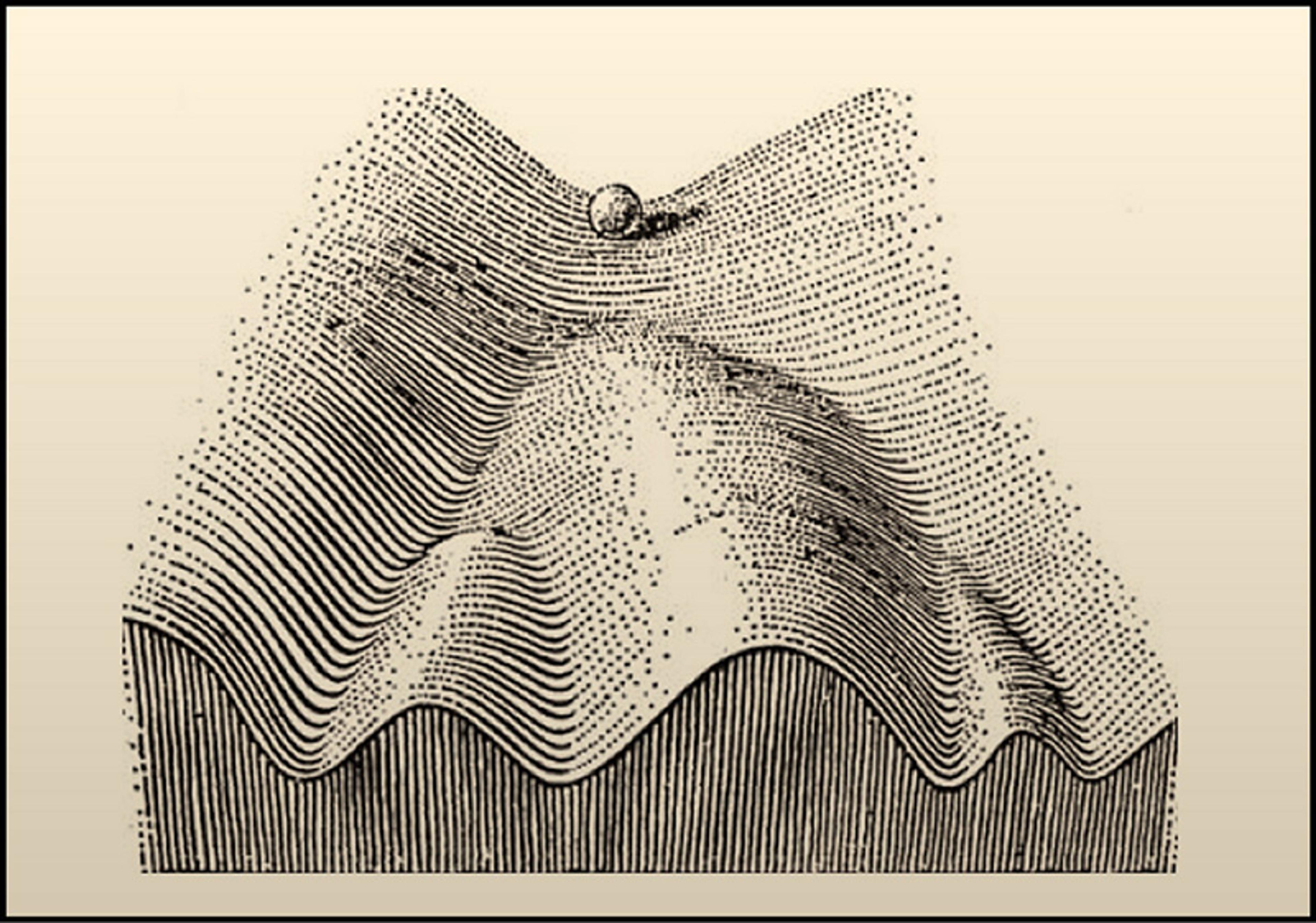
Waddington’s landscape: in the iconic metaphor for progressive development, the marble represents a cell in an embryo; as the embryo develops, the cell rolls downhill. At the first decision point, the cell might choose one of two valleys, thus becoming one of two very general cell types, for example mesoderm or ectoderm. At the next branch, the cell will become one of two very specific cell types, and so on. From The Strategy of the Genes by C H Waddington (1957) © George Allen & Unwin (London)
If the marble rolls down the valley biologists call ‘mesoderm’, it might roll further into clefts such as muscle or blood. But it’s cut off from the valleys of skin and brain, what we call ‘ectoderm’. Becoming an embryo, then, is the collective navigation of an ever-branching decision-tree by a constantly multiplying population of cells. So it’s tempting to think that some notion of sufficient complexity, a far-enough journey down the valleys, might help us divine precisely when it’s an embryo, and when it’s a human.
Edwards had studied the possibility of IVF in mice, then sheep, cows, pigs, monkeys
But, again, there’s a catch. While most cells in the early embryo rush down the valleys, a privileged few will linger at the top of the landscape. Described first in rabbits by Waddington’s own pupil at the University of Edinburgh, Robert Edwards, we now call these embryonic stem cells, and by the turn of the 21st century they were as much a part of politics as of biology. But when first described in the early 1960s, neither Edwards nor anyone else capitalised on their potential. And, anyway, Edwards was busy with another project. The era of test-tube babies was upon us.
Late in 1977, Edwards wrote a note to one of his patients, Lesley Brown: ‘[Y]ou might be in early pregnancy. So please take things quietly – no skiing.’ Some weeks earlier, she’d had one of her eggs laparoscopically inserted into her uterus; it had been fertilised in vitro with her husband John’s sperm. In 1978, Louise Brown, the first child conceived by IVF, was born.
The feat capped more than a decade of hard work. Edwards had studied the possibility of IVF in mice, then sheep, cows, pigs, monkeys. Eventually, human oocytes removed in a hospital in Oldham made the four-hour journey to Edward’s lab in Cambridge. And, there, he was the first to glimpse the moment when the Church says life begins. Coming precisely a century after Pius IX’s decision, his co-authored 1969 paper describing human fertilisation for the first time had been a watershed moment in the 3,000-year history of embryology. But it was also, well, just developmental biology: ‘Penetration of spermatozoa into the perivitelline space was first seen in eggs examined 7-7.25 h after insemination.’
The human embryo had become one of the scientists’ embryos and, in another remarkable synchronicity, the very same embryo had also exploded into the public consciousness. Not in a scientific journal, but in a glossy magazine.
The cover of Life magazine from 30 April 1965 is a startling artefact, filled by a colour photo of an 18-week human fetus. The essay inside ‘produced’ the concept of human embryos for the public just as His did for scientists during the previous century. Read by millions, it forever changed our idea of what a living, developing, growing human embryo looks like. But it was just that, an idea. In reality, the fetus on the cover of Life magazine was dead.
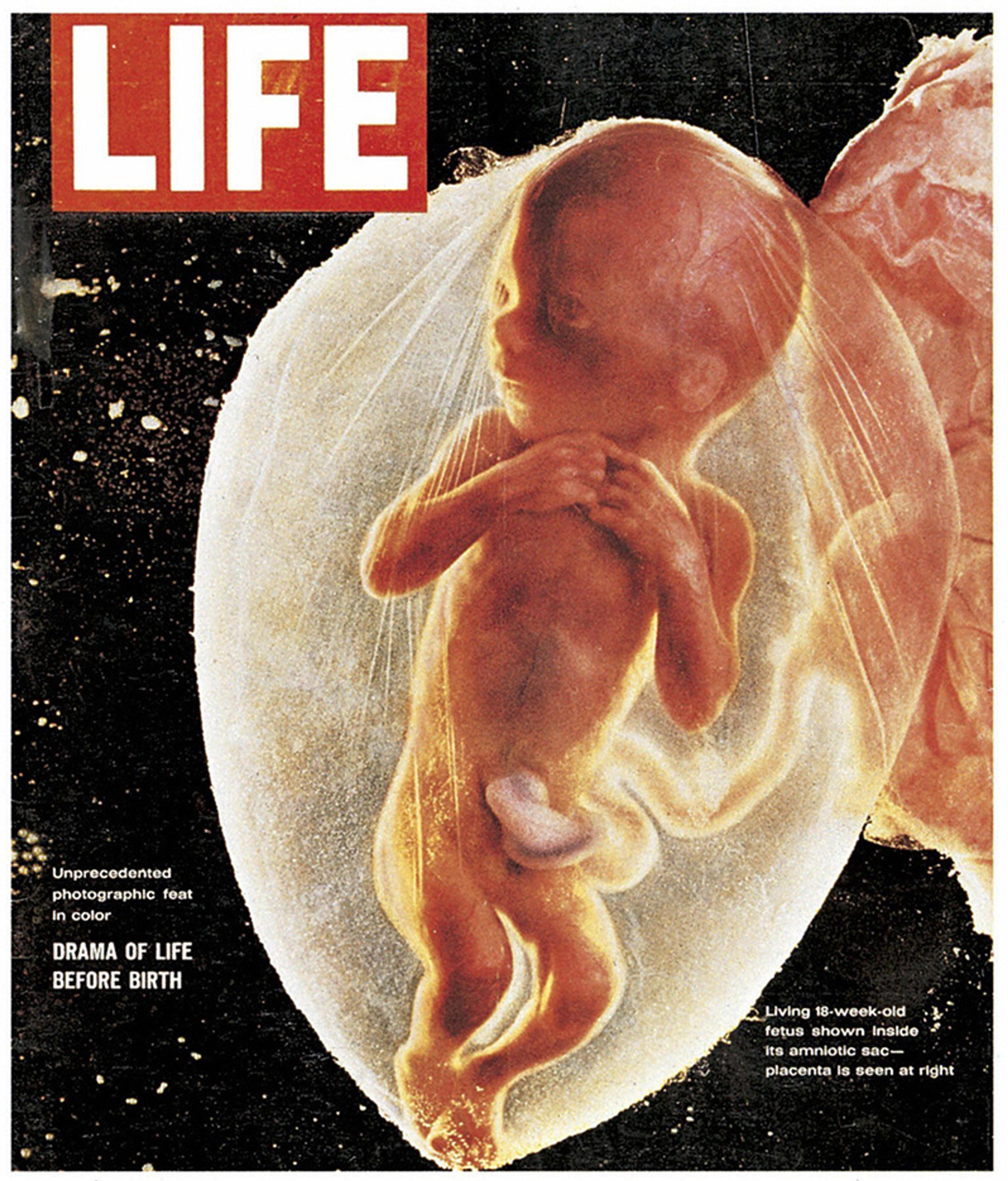
Drama of life before birth: cover of Life magazine, 30 April 1965. Courtesy Photo12/Getty
The essay was filled with similarly lifelike photos, all but one of which actually show dead or dying embryos and fetuses, the results of either miscarriage or termination. This fact was ignored by anti-abortion activists who made these images ubiquitous; it suited their needs. Depicting these surgically removed embryos as somehow both alive and autonomous made it easy to ignore the mother, whose adult body is so essential for the embryo’s growth and development, and who is so at risk. Volumes have now been written about these images and their role in the US abortion debate.
Just 77 seconds of airtime for the entire essence of development as science knows it
But what strikes the developmental biologist in me is just how accurately the essay conveyed progressive human development. We see the fertilised egg, and we follow the changes of the largely unformed embryo at three, four, and six weeks. Only at eight weeks do we finally see its gradual transition to the more obviously human fetus.
Sadly, this narrative was lost when the images were packaged into a documentary film in 1982. Influenced perhaps by Louise Brown’s birth – and that of the modern fertility industry – The Miracle of Life runs for an hour, yet the first 41 minutes show only egg or sperm. Mostly sperm. By 48 minutes, we’ve seen fertilisation, but the embryo is still just a round clump, perhaps eight cells. It’s only at 48:33 that we catch our first glimpse of the real action of development, the progressive emergence of form. And by 49:50, it’s all over. Suddenly, there are tiny fingers, eyes looking right at us. Just 77 seconds of airtime for the entire essence of development as science knows it. Shown on the BBC, PBS and outlets around the world, the award-winning documentary easily eclipsed the Life essay. The public human embryo had truly arrived – and, besides a few seconds of embryonic development shown on fast-forward, it was a fully developed fetus.
Not long after, the joyful presentations of sonograms, with their beating heart or their shadow of a face, became a core ritual of pregnancy. But these very public fetuses are wildly at odds with the biological reality of embryos, the majority of which abort spontaneously at an early stage; this led an academic theologian to muse that, if life began at fertilisation, then ‘it would appear that heaven is mostly populated by them [embryos] rather than by people who had actually been born.’
Over a scant two decades, what we now call the human embryo went from a largely intangible entity to something scientists could routinely manipulate and the public thought they understood. As the 1980s dawned, august bodies of scientists, religious leaders, lawyers and philosophers unanimously settled on a progressive view of development.
They concluded that human embryos should be kept alive in vitro only for the most important, highly regulated reproductive or research purposes. Moreover, they must be kept alive only for 14 days. This time point, chosen on the advice of a developmental biologist, was at once appropriate and arbitrary. On the one hand, it marks the onset of a process called gastrulation, by which the embryo leaves behind its early ball-like form and begins to build an elongate body. It’s also the last point at which twinning can occur, and so makes the embryo truly singular and unique. But gastrulation takes some time and embryos are variable. Only a true expert could glean the distinction between embryos at 13, 14 and 15 days. Yet, as any lawyer will tell you, laws (and even guidelines) must be specific to be meaningful, and ‘The 14-Day Rule’ was both.
Their genesis in unused embryos of IVF patients and therapeutic terminations sparked a culture war
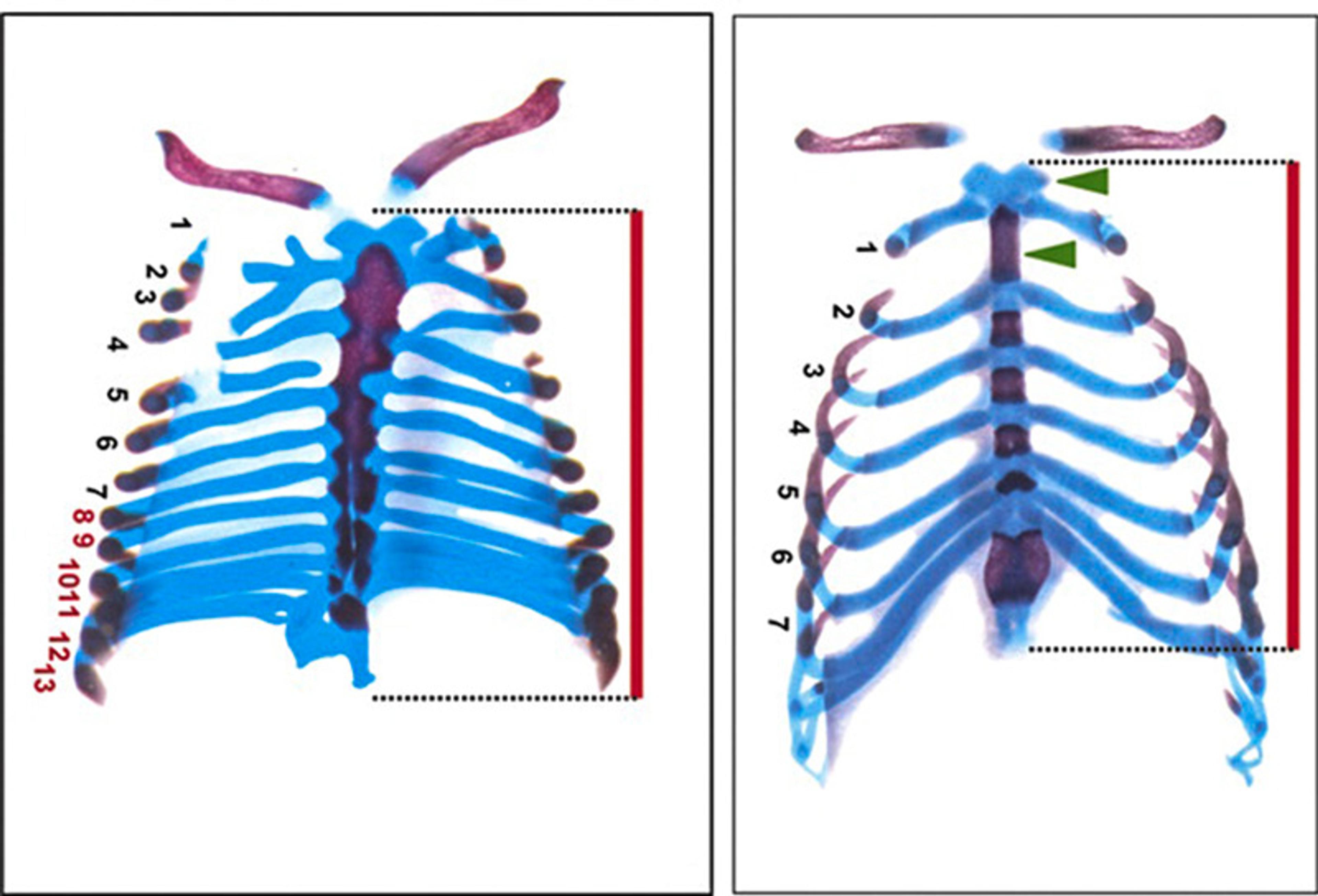
Those were exciting times for animal embryology too, given the Nobel Prize-winning work of Christiane Nüsslein-Volhard, Eric Wieschaus and Edward Lewis. They showed that the entire zoo of animals we’d studied for decades, centuries, even millennia all use a shockingly similar genetic toolkit to guide development. When chick embryos were first compared with humans in ancient Greece, it was exactly right.

A single genetic toolkit for development: flies with mutations in what scientists call homeobox genes display duplicated wings (above photo courtesy Nicolas Gompel). Mice with mutations in these genes display duplicated ribs (below, Daniel C McIntyre et al, Development [2007])
Around the same time, the biologist Gail Martin at the University of California, San Francisco made good on Edwards’s abandoned project. Coining the term ‘embryonic stem cells’, she and her colleagues learned how to get these cells from mice, keep them alive in culture dishes, and make them differentiate into cartilage or even neuron-like cells. When the same was done with human embryonic stem cells in 1998, their genesis in unused embryos of IVF patients and therapeutic terminations sparked a culture war. But neither politics nor the resulting welter of regulations dented enthusiasm for their tremendous promise – both real and as imagined by charlatans.
By tinkering with the genetic toolkit that developmental biologists discovered in animal embryos, the new stem cell scientists coaxed their wards down Waddington valleys of their choosing. Their arcane recipes recall ancient alchemy, but the ecosystems they conjured in little plastic dishes were entirely real. First, they made single human cell types, neurons, muscle, blood. Not long after, they devised functional, three-dimensional tissues, first eyes in a dish, then ‘miniguts’ and ‘minibrains’, an array we collectively call ‘organoids’.
It was only a matter of time before the idea arose that we might construct whole embryos out of stem cells. Guided by a desire to understand human development (and in some cases, surely, by at least a little hubris), progress came with unnerving speed.
At the 2022 meeting on developmental biology in Santa Cruz, I was giddy, mesmerised by the confluence of developmental and stem cell biology. Lehmann’s lecture on flies and my own about frogs joined others about fish and worms. There was even a lecture about jerboas, a strange hopping rodent from Mongolia. One talk really blew my mind: unable to study rhinoceros embryos, for obvious reasons, one group has convinced their stem cells to make rhino embryo models of a sort.
My joy, however, soon bled into dismay when The Washington Post, describing the mouse embryo models developed by Hanna and by Żernicka-Goetz, noted rightly that human models were all but inevitable. Given that years of debate went into the 14-Day Rule in the 1980s, we might have expected that move to be cautious and deliberate. It wasn’t. At a conference in Boston in June 2023, Żernicka-Goetz claimed that ‘we can create human embryo-like models by the reprogramming of [embryonic stem] cells’, a statement The Guardian blasted out to the public the following day without any back-up from the peer review. Once the peer-reviewed paper appeared, it became clear that Żernicka-Goetz’s initial claim had been overstated. Hanna’s group reported more impressive human embryo models soon after, but these couldn’t justify the media commentary either.
The work, while vetted and approved by the appropriate ethics committees, is a far cry from helping us frame the ethical considerations these embryo models will raise. Indeed, while the current embryo models cannot develop into a viable fetus, it sure looks like we will get to that point. And it doesn’t help that the International Society for Stem Cell Research in 2021 relaxed the 14-Day Rule for research with human embryos made the old-fashioned way. Unlike the careful deliberation with stakeholders in the 1980s, the new decision was reached without public engagement. I think the entire field is obligated to bring more people into the conversation and to better articulate why the work is necessary – why, in fact, we must make human embryos from scratch.
This science has always been a proxy, however imperfect, for understanding how our own bodies come to be
It’s troubling, too, that the scientists getting the most attention don’t always use their cachet to communicate the nuance, both ethical and biological. Instead, it’s left to others. Alfonso Martinez Arias, Nicolas Rivron and Kathy Niakan, for example, are among those who have provided thoughtful commentary on the complexities in scientific journals. And, while Żernicka-Goetz in June 2023 told The New York Times that ‘we do it to save lives, not create it’, the medical applications are not at all clear to me. Exactly how will these models save lives? And exactly how do they compare with alternative solutions to the problem? Without such details, how can we weigh what’s to be gained against our ethical and moral obligations?
By contrast, the decades of research with old-fashioned human embryos, all conducted within the confines of the 14-Day Rule, brought us a remarkably safe and effective fertility industry, as well as important advances in genetic diagnosis and prevention of diseases and birth defects. These advances continue, with benefits that are clear.
We’ve pondered embryos for thousands of years, in part because they spark our inherent wonder; theirs is the ultimate emergent property. Across that long arc, it’s usually been animal embryos under our microscopes, organisms that assemble themselves just like we do but whose development we have fewer qualms about interrupting for the sake of knowledge. Like any basic science, animal embryos provide ‘a glimpse of what is possible in this world’, Lehmann writes. But this science has always been a proxy, however imperfect, for understanding how our own bodies come to be. And, quite suddenly now, we seem to have the tools and the appetite to get far more than just a glimpse at the human embryo.
Martinez Arias recently told me that ‘when you put the word “human” there, you are talking to the whole of society.’ It’s worth recalling, then, that this conversation is also thousands of years old. And history tells us that our collective decisions on issues of the human embryo will ultimately be influenced by both science and faith.
Science can tell us how the human embryo develops, and it is an undisputed certainty that embryos develop progressively, building complexity and identity only over time. But there is no scientific consensus on when during that progression ‘life’ begins. Likewise, there is no consensus among faiths on when life begins. Certain Christian faiths now hold that life begins at conception, and these have an outsized influence. Yet, even within Christianity, that view is a recent stance, and one that reversed centuries of thought. Other Western religious traditions don’t share Christianity’s ambiguity. Cleaving to the ancient gradualist view of development, Islamic tradition generally holds the embryo to become human 120 days after fertilisation, though some use the 40-day mark; in most Jewish traditions, it happens only at birth.
We are 3,000 years deep in the adventure called developmental biology, yet the embryo remains in many ways just as mysterious as ever. As we enter a new era of explicitly human developmental biology, we should approach it with all the grace and humility we possibly can.